The 2015 Annual Meeting of the American Academy of Otolaryngology–Head and Neck Surgery was just held in Dallas from September 27-30. Although I typically give several talks related to sleep apnea surgery, whether focused on drug-induced sleep endoscopy or sleep apnea procedures related to the Palate Region or Tongue Region, one of the most enjoyable and interesting parts of the conference is sharing experiences and findings with colleagues. This occurs informally as well as during formal sessions devoted to research presentations. This year, there were a number of interesting research studies that were presented.
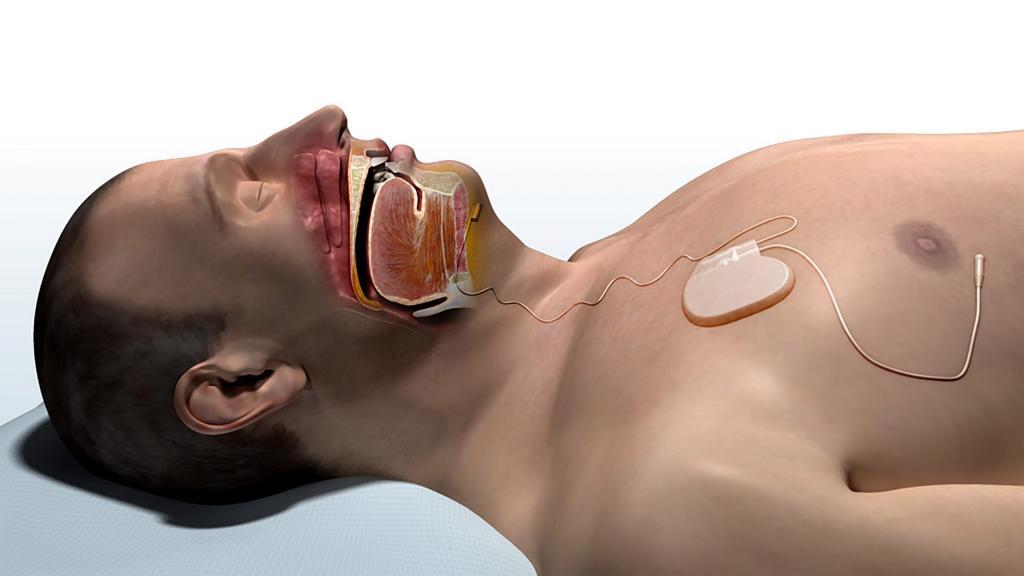
Dr. Ryan Soose from the University of Pittsburgh spoke about the STAR Trial that demonstrated the benefits of the Inspire Upper Airway Stimulation system for treatment of obstructive sleep apnea. The study screened over 1000 individuals who were unable tolerate positive airway pressure therapy (such as CPAP). Interestingly, many of them initially did well enough with positive airway pressure therapy to keep trying, as only 28% abandoned it completely within a year, but the problem was that more people were unable to continue with it as time passed. The same was true for oral appliances. 18% of the individuals screened in the STAR Trial had attempted an oral appliance; 35% of these individuals failed within a year, with more rejecting it over time (potentially related to some of the side effects that can become more problematic with continued use). Of note, 17% of those screened had previous surgery and still had sleep apnea. The key finding of the study was that patients with sleep apnea need to recognize that a treatment only works if they achieve good results and if they can use it (for CPAP and oral appliances).
One of our current fellows, Dr. David Kent, then spoke about the University of Pittsburgh experience with Upper Airway Stimulation following approval by the United States Food and Drug Administration. Many medical drugs and devices obtain this approval, and the results that follow are not as good as were seen in the studies done before FDA approval. Importantly, the Pittsburgh experience is that the results were just as good, if not better. Almost all (95%, or 19/20) of their patients had marked improvement or resolution of their sleep apnea. In addition, patients were comfortable using it, as the average nightly use was 7 hours, explaining the major improvements in the level of sleepiness (a decrease from 10.3 to 6 in something called the Epworth Sleepiness Scale score). At Keck Medicine of USC, we have had similar results that seem even better than the STAR Trial, and we are looking forward to starting our enrollment in the study of patients now receiving the Upper Airway Stimulation system that was required by FDA as part of the approval.
Dr. Andrew Baker, in collaboration with Dr. Boyd Gillespie at the Medical University of South Carolina, presented an analysis of the American College of Surgeons’ National Surgical Quality Improvement Program database examining complications after sleep apnea surgery. The question we often ask is whether combining multiple procedures, for example Palate Region Surgery and Tongue Region Surgery procedures, together increases the risks of complications compared to performing the sleep apnea procedures separately. They showed that there was no increased risk of complications, although patients did stay in the hospital longer after the combination of procedures. With Dr. Ed Weaver from the University of Washington, we performed two studies showing that the risk of serious complications was increased, but it was not possible to know whether the risk when the procedures were combined were higher than for adding up the risk for two procedures performed separately.
I have been fortunate to work with Dr. Hsueh-Yu Li from Chang Gung Memorial Hospital in Taiwan. Hsueh-Yu has developed some sleep apnea procedure techniques, including relocation pharyngoplasty and Coblation Endoscopic Lingual Lightening (CELL). I have been a coauthor on the publication of the CELL technique, showing that it is a systematic approach to tongue base resection in sleep apnea patients that can simplify this approach without requiring expensive equipment. In Dallas, Hsueh-Yu presented results he has achieved when combining relocation pharyngoplasty and CELL, yielding an improvement compared to a similar group of patients in whom he performed relocation pharyngoplasty alone.




− 9 = 1